Abstract
Background: Monoclonal antibodies (MoAb) including daratumumab (Dara) and elotuzumab (Elo) are important additions to multiple myeloma (MM) therapeutics and have become cornerstones of patients with relapsed and/or refractory disease (RRMM). While large clinical trial data define their efficacy and safety, real-world experience to help understand their true utilization and serve as guidelines for community practice are lacking.
Methods: Data on the utilization of Dara and Elo for treatment of MM including patient and disease characteristics, infusion attributes, supportive medication use and adverse event profile were collected and analyzed using descriptive statistics. Baseline laboratory data including median absolute lymphocyte count (ALC), eosinophil count (AEC), neutrophil count (ANC) and immunoglobulin level (Ig) were documented and their correlation with infusion-related reactions (IRRs) was explored using Wilcoxon rank sum test.
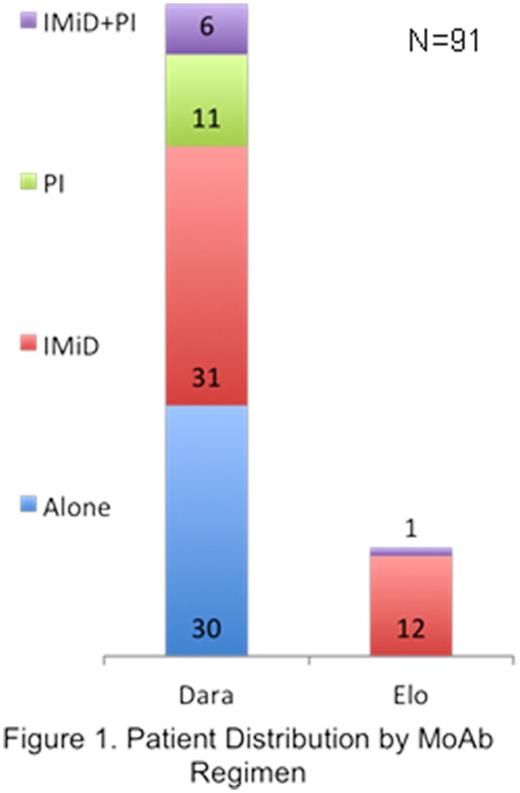
Results: Ninety-one consecutive patients with MM treated using Dara (n=78) or Elo (n=13) not on clinical trials between 1/1/16 and 12/31/16 at Mayo Clinic in Florida (MCF; n=30) or Arizona (MCA; n=61) were included. A total of 1036 infusions of Dara (Median: 12 per patient; Range: 1-38) and 210 of Elo (Median: 18 per patient; Range: 3-29) were administered over 12 months. Study cohort included 56 males (61.5%) with median age 63 years (range: 37-81) and majority (83.5%) of Caucasian race. Treatment regimes of MoAb alone or with immunomodulatory drug (IMiD) and/or proteasome inhibitor (PI) are shown in Figure 1. Prior lines of therapy were >3 in 52.7%, 3 in 13.2%, 2 in 19.8%,1 in 12.1% and in 1 case each Dara and Elo were given in first line of therapy. Majority of patients who received Dara (60.3%) had >3 prior lines of therapy while majority who received Elo (53.8%) had 1 prior line of therapy for MM. Pre-treatment blood sample for type/screen was sent in 67% patients. Any grade hematologic (heme) adverse events (AE) were seen in 66 (84.6%) patients with Dara and 6 (46.2%) patients with Elo, while non-heme AEs were seen in 64 (82.1%) patients with Dara and 11 (84.6%) patients with Elo. Actual MoAb administration took an average 2 hours lesser (Range: -3 to +6 hours for Dara and -1 to +5 hours for Elo) than scheduled with median 2 hours lesser for both the drugs. In 2 cases first Dara infusion was given in divided dose over 2 days. Most common premedications prior to first MoAb infusion included acetaminophen (90.1% cases), diphenhydramine (86.8% cases) and methylprednisone (78% cases) with dexamethasone, famotidine, prochlorperazine and ondansetron used less frequently. Number of patients without any premedications use changed from 9.9% before infusion 1 to 94.5% before infusion 29 of any MoAb, showing a sharp decrease in the use of premedications in subsequent dose infusions of MoAbs. IRRs were seen in 39 (42.9%) patients with first MoAb infusion with respiratory IRRs (bronchospasm, cough, dyspnea, nasal congestion, throat irritation, wheezing) seen in 35 (38.5%) and non-respiratory IRRs (chills, nausea, hypertension, tachycardia, pruritus, bradycardia, emesis) in 16 (17.6%) patients. No patients had any higher than grade 2 IRRs. Majority of the IRRs (91.3%) were observed during the first MoAb infusion, none of the patient's required discontinuation of MoAb treatment as a result of IRRs, with a significant decrease in IRR incidence during second (3.3%) and subsequent (5.4%) infusions. In 10 cases (9 for Dara and 1 for Elo) premedication regimen was modified after first infusion. MoAb AEs seen in ≥10% cases across various regimens included neutropenia (51.6%), lymphopenia (42.9%), thrombocytopenia (33%), anemia (18.7%), fatigue (40.7%), diarrhea (22%), pneumonia (22%), cough (18.7%), fever (18.7%) and nausea (12.1%). Median baseline labs included ALC 0.95 K/mm3, ANC 2.22 K/mm3 and AEC 0.07 K/mm3. There was no association seen between median baseline ALC, ANC or AEC and the risk of any IRRs, respiratory or non-respiratory.
Conclusions: We present a comprehensive analysis of real-world experience regarding the utilization of novel MoAb agents for the treatment of MM. Although the role of MoAbs in MM therapeutics is evolving, these data help understand their true utilization and identify how resources may be best employed to optimize their benefit for patients.
Ailawadhi: Novartis: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Pharmacyclics: Research Funding; Amgen: Consultancy, Honoraria. Sher: LAM Therapeutics, Inc: Research Funding. Fonseca: Bayer: Consultancy; Takeda: Consultancy; Jansen: Consultancy; Celgene Corporation: Consultancy, Research Funding; AMGEN: Consultancy; Bristol-Myers Squibb: Consultancy; Mayo Clinic & Dr Fonseca: Patents & Royalties: Prognostication of myeloma via FISH, ~$2000/year; Merck: Consultancy; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy; Novartis: Consultancy; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal